CRISPR/Cas9 Gene Editing: Revolutionizing Autoimmune Disease Treatment
CRISPR gene editing offers new hope for patients with autoimmune diseases, providing targeted and precise genetic modifications that traditional treatments cannot achieve.
How Does CRISPR Technology Work?
Clustered regularly interspaced palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) is a groundbreaking gene-editing tool revolutionizing the treatment of various autoimmune diseases by altering DNA sequences and modifying genes.
CRISPR works like this: it uses a guide RNA (gRNA) to lead a protein called Cas9 to a specific spot in the DNA. Cas9 acts like a pair of molecular scissors, cutting the DNA at that spot. (1,2)
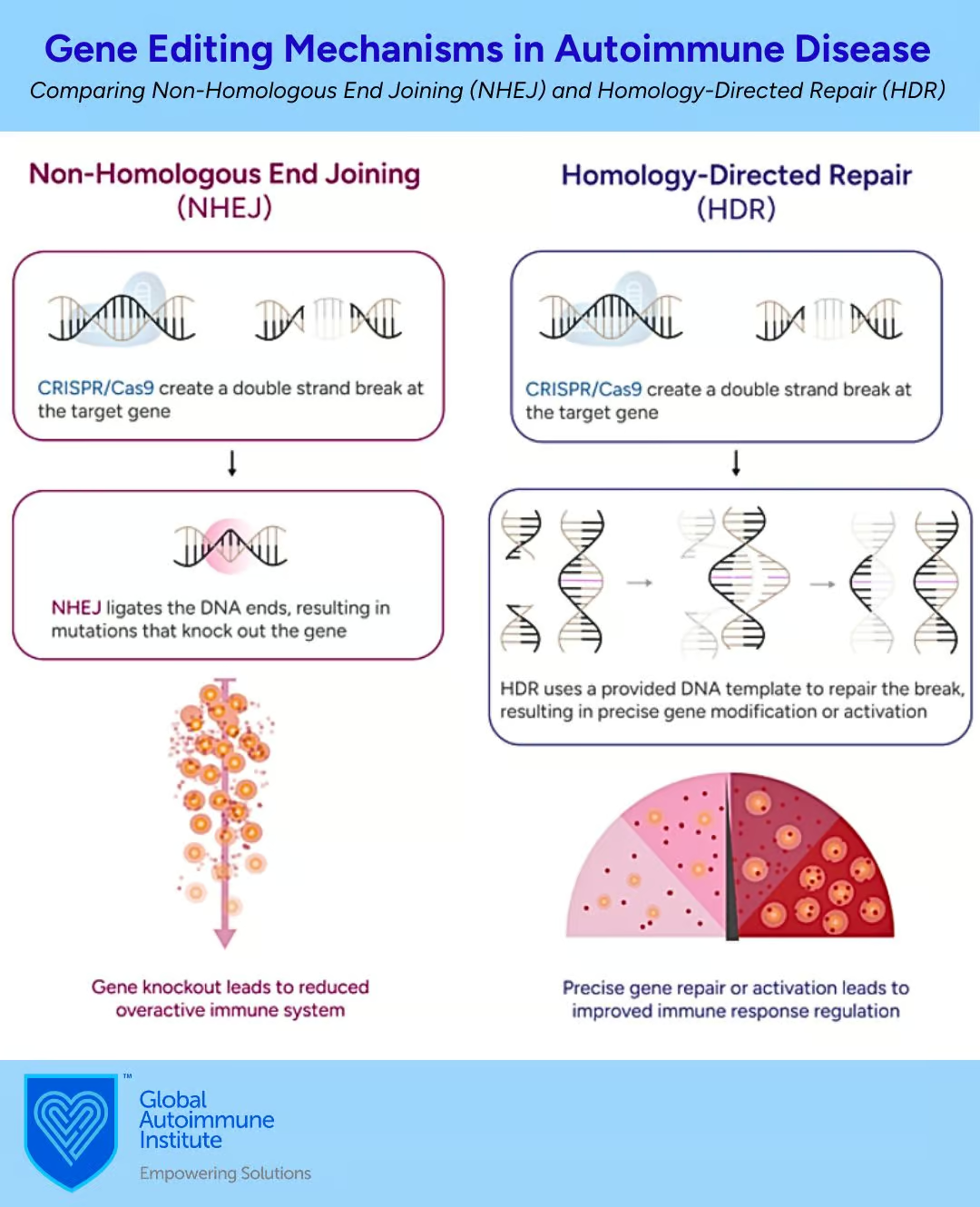
Once the DNA is cut, the cell tries to repair it using two different methods: non-homologous end joining (NHEJ) or homology-directed repair (HDR). (3)
What Are the DNA Repair Mechanisms Activated by CRISPR/Cas9?
Non-homologous end joining (NHEJ):
- The broken DNA ends are quickly joined together (through ligation). This process is not very precise and can often result in changes to the DNA, such as mutations, deletions, or insertions. These changes can sometimes disable the gene, effectively turning it off. Because this method is less accurate, it can sometimes add random, ‘nonsensical’ genetic material.” (4)
- Often used for creating gene knockouts by introducing small insertions or deletions that disrupt the gene’s function. This is useful for studying gene function or creating model organisms.
- Works in all cell types and throughout the cell cycle, including non-dividing cells.
Homology-directed repair (HDR):
- Uses a matching piece of DNA as a guide (a homologous DNA template) to fix the break accurately. This method allows scientists to make precise changes, like fixing a broken gene or adding a new one, and can even reactivate a gene that was turned off. (3)
- More effective in dividing cells and during the S and G2 phases of the cell cycle when a homologous template is available.
What Is the History of CRISPR Technology?
CRISPR technology is both powerful and easy to use. It all started in 1987 when researchers noticed a repetitive DNA sequence in a bacterium called Escherichia coli. This discovery suggested that bacteria could remember and target specific viral DNA sequences. Since then, many studies have advanced gene editing significantly.
How Does CRISPR Modify Genes Associated with Autoimmune Disorders?
Many autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, type 1 diabetes mellitus, and multiple sclerosis, have a strong genetic component. As a result, gene therapy has promising applications.
Autoimmune diseases are often treated with immunosuppressive drugs meant to control an immune response. But CRISPR/Cas9 offers a targeted approach to modify specific genes implicated in autoimmune disorders.
In addition to being a treatment option for infectious diseases and cancers, researchers have begun examining the technology to learn about the mechanisms of pathogenesis and possible treatments for ADs. (6, 2)
What Are the Potential Applications of CRISPR in Autoimmune Disease Treatment?
The following section provides a summary of two recent studies which reviewed advances related to CRISPR/Cas9 in infection and autoimmunity. Those studies are:
“Genome Editing Using CRISPR-Cas9 and Autoimmune Diseases: A Comprehensive Review” (7) and “CRISPR/Cas9 gene editing: A new therapeutic approach in the treatment of infection and autoimmunity.” (2)
For more information on autoimmune disease treatments, visit our Autoimmune Disease Treatments page.
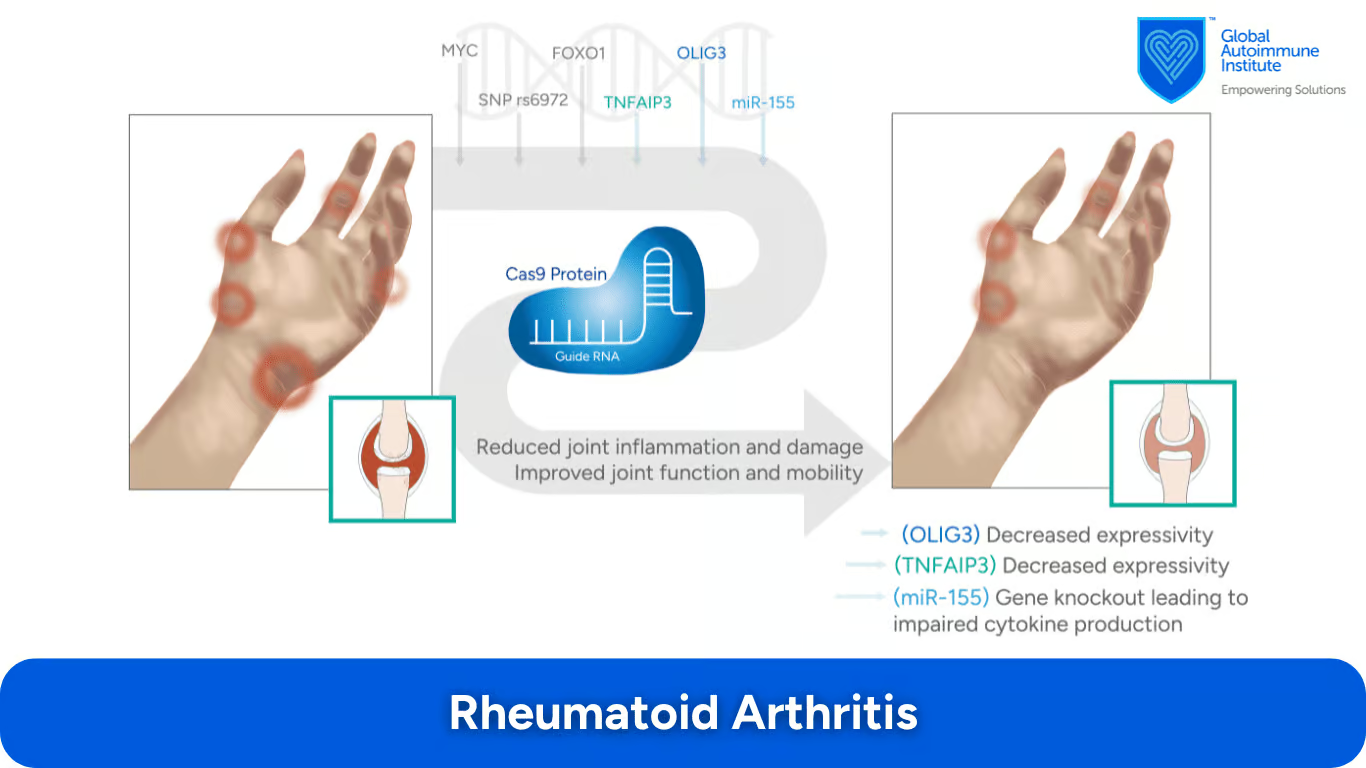
How Can CRISPR/Cas9 Help in Treating Rheumatoid Arthritis (RA)?
In RA, the immune system attacks the joints, causing inflammation. According to Min Ho Lee et al., the MYC, FOXO1 gene, SNP rs6927172, TNFAIP3, OLIG3 gene, and miR-155 may all be relevant for CRISPR/Cas9 treatment. (7):
These genes have been found to be possible causal factors of RA.
An intergenic SNP on chromosome 6q23 region is associated with RA.
Using CRISPR/Cas9, these genes showed decreased expressivity in a specific genomic region.
Turning off this microRNA reduces the production of pro-inflammatory cytokines, playing an important role in RA.
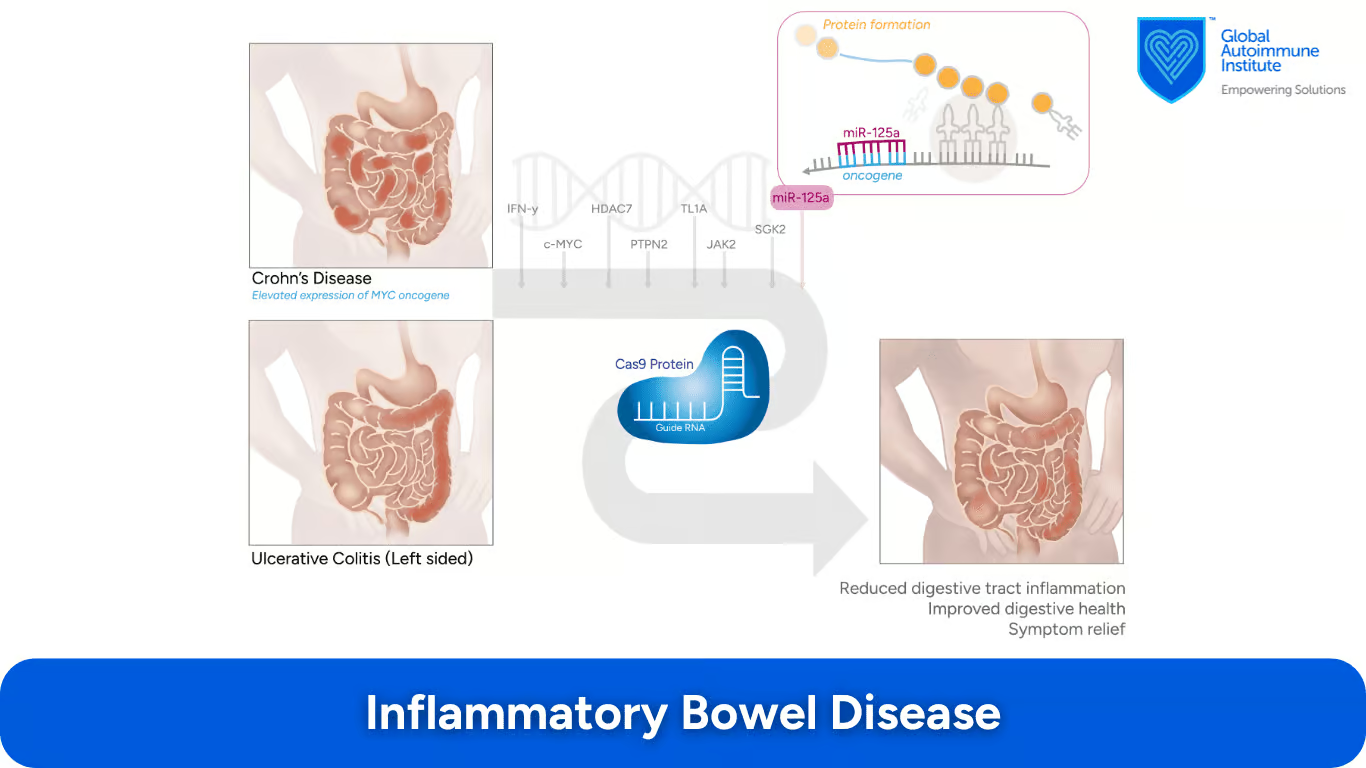
What Are the CRISPR/Cas9 Applications in Inflammatory Bowel Disease (IBD)?
IBD includes Crohn’s disease and ulcerative colitis, which involve chronic inflammation of the digestive tract. (8) According to Min Ho Lee et al., JAK2, TL1A, SGK2, PTPN2, c-MYC, HDAC7, and IFN- γ genes and miR-125a may all be candidates for CRISPR-Cas9 technology:
rs1887428 in the promoter region of the Janus kinase 2 [JAK2] gene, as well as SGK2, were found to potentially impact the course of IBD.
Elevated expression levels were observed in Crohn’s disease patients.
IFNγ has been found to play a key role in colon inflammation
Flare-ups of these genes are noted in IBD (10)
Reduced function of this gene, Histone deacetylase 7 (HDAC7) helps maintain the intestinal barrier in IBD patients (10)
This microRNA protects against intestinal inflammation, with knockout mice showing more severe colitis (11)
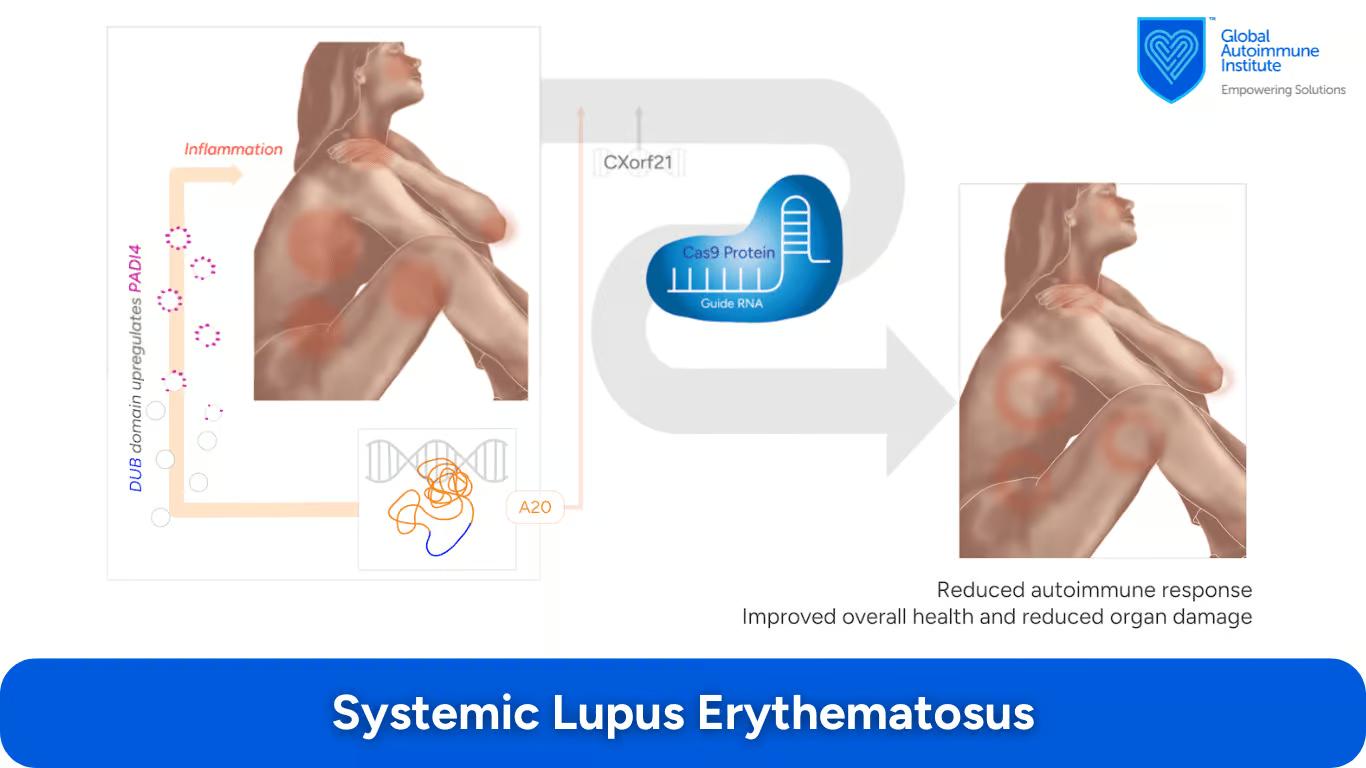
How Does CRISPR/Cas9 Target Genes in Systemic Lupus Erythematosus (SLE)?
The A20 DUB and CXorf21 gene have both been suggested as appropriate candidates for CRISPR-Cas9 treatment.
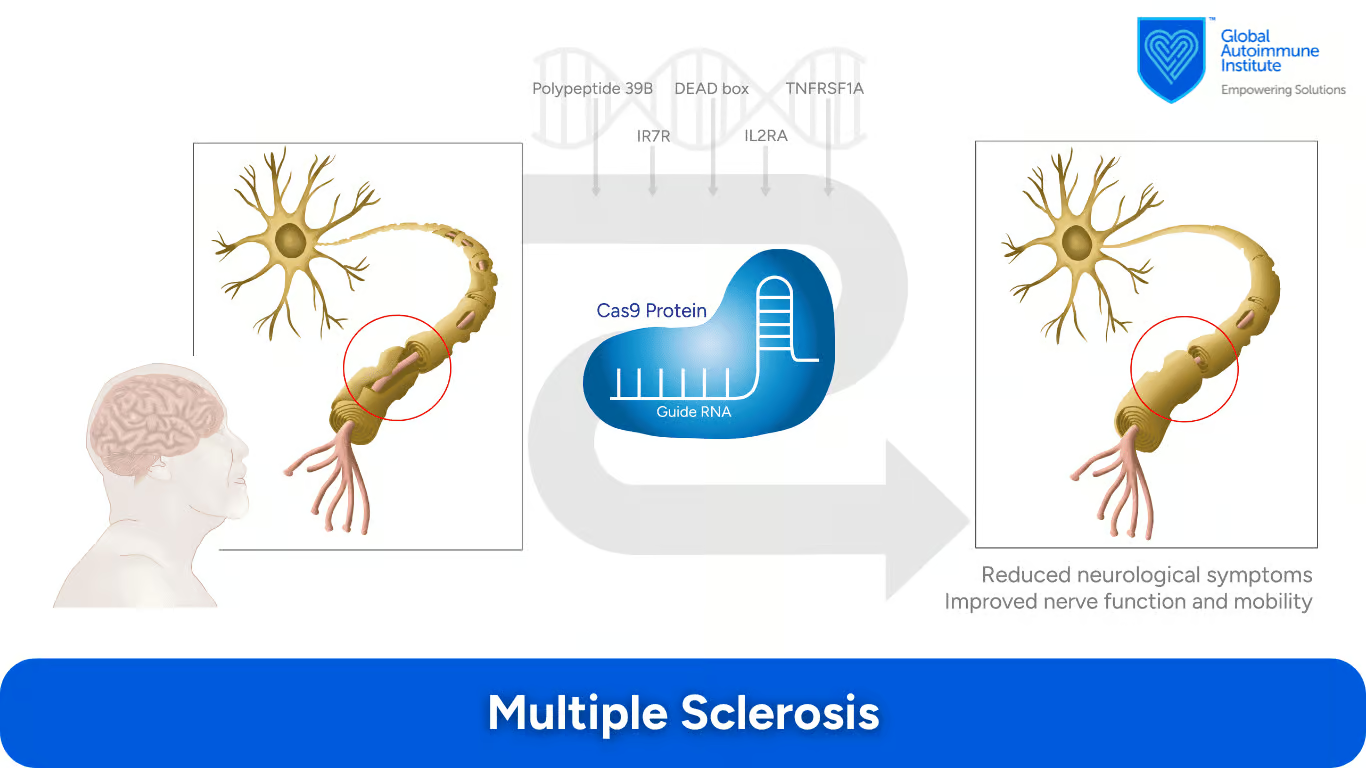
Can CRISPR/Cas9 Be Used to Treat Multiple Sclerosis (MS)?
According to the review by Min Ho Lee et al., the IR7R gene, the RNA helicase DEAD box polypeptide 39B, the IL2RA gene, and the TNFRSF1A gene may all be appropriate candidates for CRISPR-Cas9 treatment.
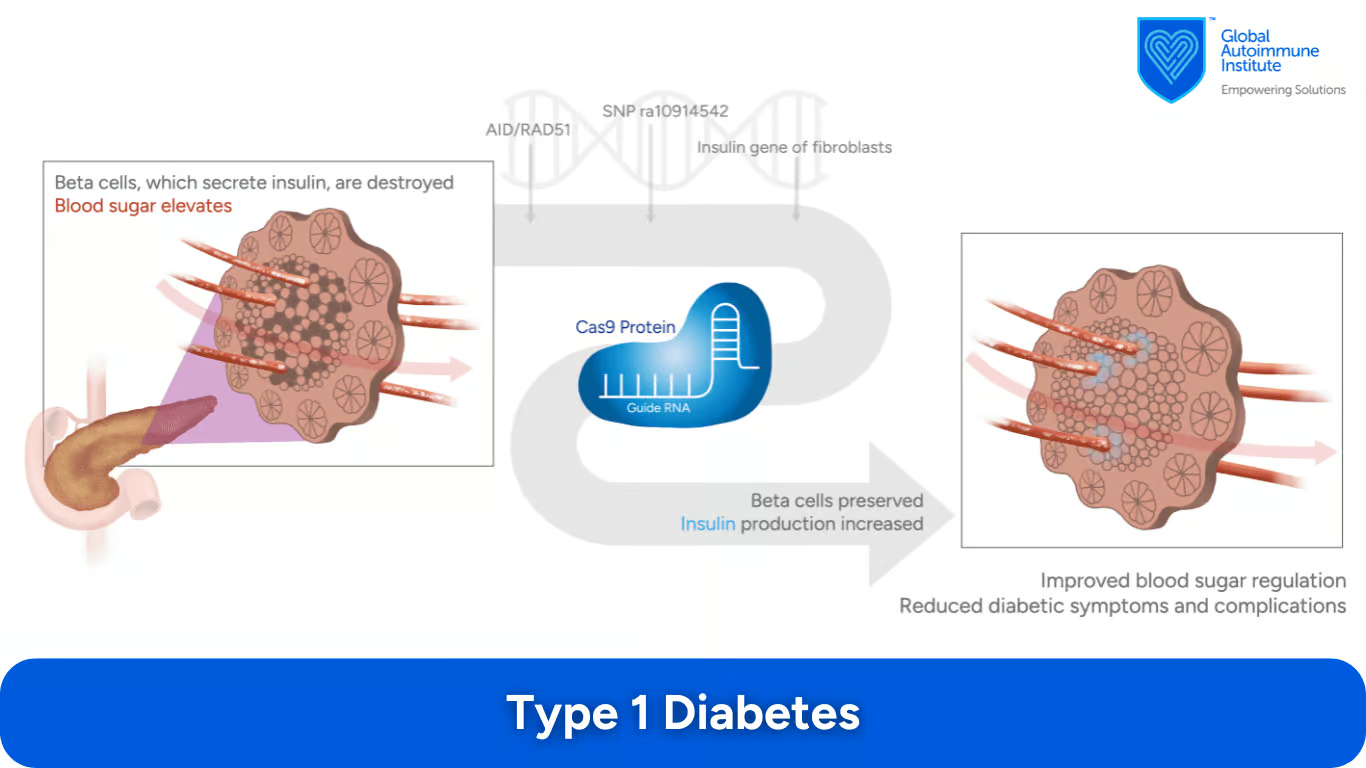
What Is the Role of CRISPR/Cas9 in Treating Type 1 Diabetes?
In Type 1 Diabetes Mellitus (T1DM), the body’s immune system attacks pancreatic B cells, causing the body to produce insufficient insulin. The AID/RAD51, and SNP rs10914542 of LCK gene have been suggested as appropriate candidates for CRISPR-Cas9 treatment.
found to be a potential target for treatment of T1DM patients (16)
SNP rs10914542 of the LCK gene was significantly associated with the susceptibility of T1DM (17)
a CRISPR/Cas9 system fused with transcriptional activators (dCas9-VP160) has been found to activate the transcription of the insulin gene in T1DM patient’s derived fibroblasts (18)
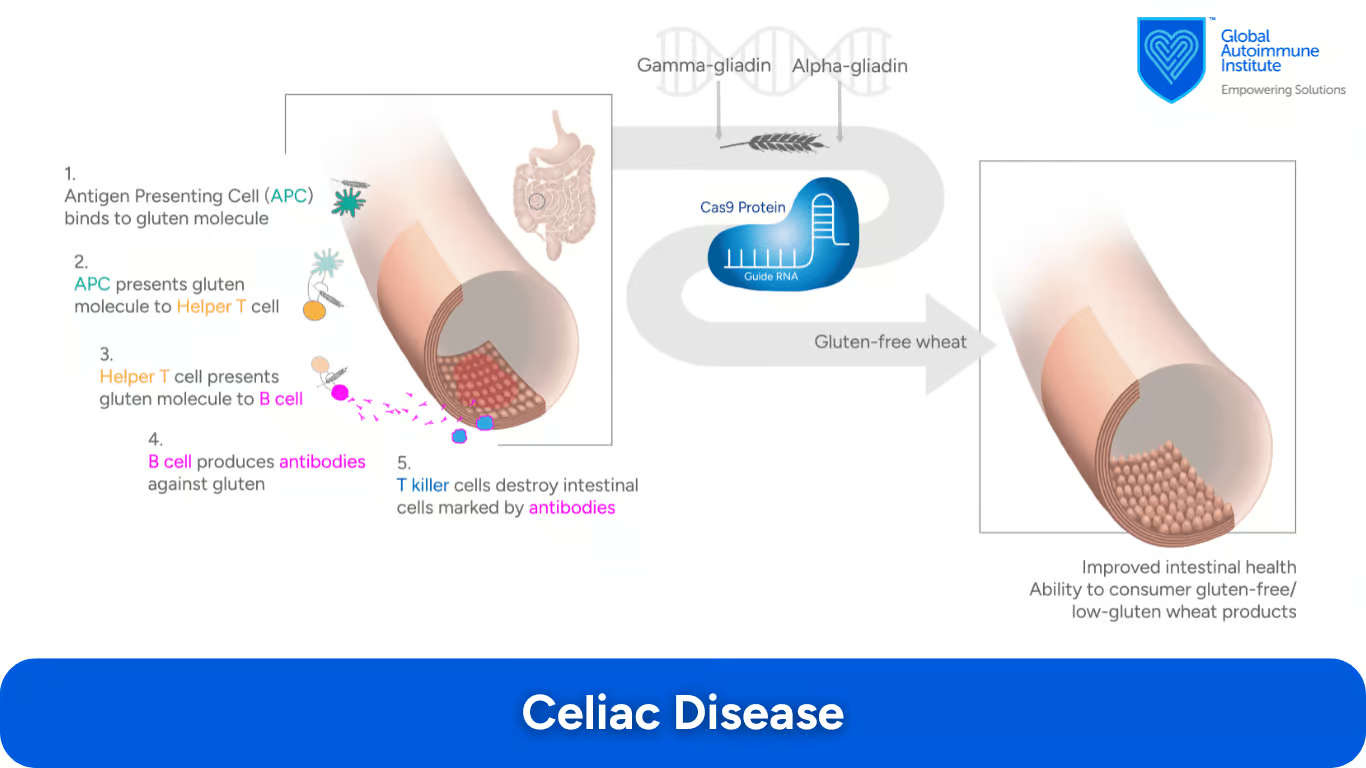
How Can CRISPR/Cas9 Technology Help in Celiac Disease (CD)?
Celiac Disease is an autoimmune disorder which causes damage to the small intestine as a result of inflammatory reactions induced by gluten. The α- or γ-gliadin genes (gluten encoding genes) have been suggested as candidates for CRISPR-Cas9 treatment.
In one study, CRISPR-Cas9 was shown to successfully edit α- or γ-gliadins and make gluten-free or low-gluten wheat. (19)
What Are the Limitations of CRISPR in Autoimmune Disease Treatment?
CRISPR/Cas9 technology is a promising method for understanding and treating autoimmune diseases. CRISPR also exhibits higher efficiency and fewer off-target effects than other gene editing tools such as ZFNs and TALENS. (4) However, CRISPR-Cas systems are frequently unreliable in editing primary cells and certain tissues, and in patient’s bodies. One major limitation is effectively directing Cas9 to the desired sequence. Developing more effective editing techniques is crucial for advancing human gene therapy applications. (4, 6)
What Are the Promising Preclinical Studies on CRISPR/Cas9 for Autoimmune Diseases?
According to Çerçi et al, there have been advances which have successfully resulted in “increased survival of model animals and tolerable safety.“ (20)
That team has listed some research that is close to the clinical phase here.
Promising advancements related to CRISPR/Cas9 gene editing have been made in the last four decades. While much remains to be accomplished, CRISPR/Cas9 technology provides a way to manipulate the genome and marks a significant advance for diseases and illnesses of many kinds.

Sources
- Article Sources
Redman, M., King, A., Watson, C., & King, D. (2016, August 1). What is CRISPR/cas9? ADC Education & Practice Edition. https://ep.bmj.com/content/101/4/213.short
Mohammadzadeh, I., Qujeq, D., Yousefi, T., Ferns, G. A., Maniati, M., & Vaghari-Tabari, M. (2020). CRISPR/Cas9 gene editing: A new therapeutic approach in the treatment of infection and autoimmunity. IUBMB Life, 72(8), 1603-1621. https://doi.org/10.1002/iub.2296
Swati, T., Kumar, R., Das, A., Won, S. Y., & Shukla, P. (2020). CRISPR-Cas9 system: A genome-editing tool with endless possibilities. Journal of Biotechnology, 319, 36-53. https://doi.org/10.1016/j.jbiotec.2020.05.008
Alagoz, M., & Kherad, N. (2020). Advance genome editing technologies in the treatment of human diseases: CRISPR therapy (Review). International Journal of Molecular Medicine, 46(2), 521-534. https://doi.org/10.3892/ijmm.2020.4609
Ishino, Y., Krupovic, M., & Forterre, P. (2018). History of CRISPR-Cas from Encounter with a Mysterious Repeated Sequence to Genome Editing Technology. Journal of Bacteriology, 200(7), e00580-17. https://doi.org/10.1128/JB.00580-17
Wenyi, L., Luoxi, L., Jianxin, J., Min, W., & Ping, L. (2021). Applications and challenges of CRISPR-Cas gene-editing to disease treatment in clinics. Precision Clinical Medicine, 4(3), 179-191. https://doi.org/10.1093/pcmedi/pbab014
Lee, M. H., Shin, J. I., Yang, J. W., Lee, K. H., Cha, D. H., Hong, J. B., Park, Y., Choi, E., Tizaoui, K., Koyanagi, A., … & Jacob, L. (2022). Genome Editing Using CRISPR-Cas9 and Autoimmune Diseases: A Comprehensive Review. International Journal of Molecular Sciences, 23(3), 1337. https://doi.org/10.3390/ijms23031337
Moein, S., Vaghari-Tabari, M., Qujeq, D., Majidinia, M., Nabavi, S. M., & Yousefi, B. (2019). MiRNAs and inflammatory bowel disease: An interesting new story. Journal of Cellular Physiology, 234(3), 3277-3293. https://doi.org/10.1002/jcp.27173
Matthews, S. M., Eshelman, M. A., Berg, A. S., Koltun, W. A., & Yochum, G. S. (2019). The Crohn’s disease associated SNP rs6651252 impacts MYC gene expression in human colonic epithelial cells. PLoS ONE, 14(2), e0212850. https://doi.org/10.1371/journal.pone.0212850
Friedrich, M., Ganther, J., Breiderhoff, T., Rosenthal, R., Glauben, R., & Siegmund, B. (2017). P063 HDAC as versatile regulators of the intestinal epithelial barrier in inflammatory bowel disease. Journal of Crohn’s and Colitis, 11(Suppl 1), S109. https://academic.oup.com/ecco-jcc/article/11/suppl_1/S109/2961007
Ge, Y. D., Sun, M. M., Wu, W., Ma, C. Y., Zhang, C., He, C., Li, J. X., Cong, Y. Z., Zhang, D. K., & Liu, Z. J. (2019). MicroRNA-125a suppresses intestinal mucosal inflammation through targeting ETS-1 in patients with inflammatory bowel diseases. Journal of Autoimmunity, 101, 109-120. https://www.sciencedirect.com/science/article/pii/S0896841119300678?casa_token=1WkQCNN0K8wAAAAA:P_Aec6Z5tTEo-MRxROBoDWjAV8ypcMwYqyqCv2ZUbCrrcNMFB1nurGasZLWi9dwDh-FgDixN5Ew
Odqvist, L., et al. (2019). Genetic variations in A20 DUB domain provide a genetic link to citrullination and neutrophil extracellular traps in systemic lupus erythematosus. Annals of the Rheumatic Diseases, 78(10), 1363-1370. https://ard.bmj.com/content/78/10/1363.abstract
Harris, V. M., Harley, I. T. W., Kurien, B. T., Koelsch, K. A., Scofield, R. H., McMahon, F. J., & James, J. A. (2019). Characterization of CXorf21 provides molecular insight into female-biased immune response in SLE pathogenesis. Frontiers in Immunology, 10, 2160. https://doi.org/10.3389/fimmu.2019.02160
Maier, L. M., Lowe, C. E., Cooper, J., Downes, K., Anderson, D. E., Severson, C., Clark, P. M., Healy, B., Walker, N., Aubin, C., … & Todd, J. A. (2009). IL2RA genetic heterogeneity in multiple sclerosis and type 1 diabetes susceptibility and soluble interleukin-2 receptor production. PLoS Genetics, 5(1), e1000322. https://doi.org/10.1371/journal.pgen.1000322
Gregory, A. P., Dendrou, C. A., Attfield, K. E., Haghikia, A., Xifara, D. K., Butter, F., Poschmann, G., Kaur, G., Lambert, L., Leach, O. A., Prömel, S., Tuku, B., Lahmann, H., Paavola, J., Rice, C. M., Compston, A., Leslie, S., Gnanapavan, S., Marta, M., … & Fugger, L. (2012). TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature, 488(7412), 508-511. https://www.nature.com/articles/nature11307
Ratiu, J. J., Racine, J. J., Hasham, M. G., Wang, Q., Branca, J. A., Chapman, H. D., Zhu, J., Donghia, N., Philip, V., Schott, W. H., … & Zhu, J. (2017). Genetic and small molecule disruption of the AID/RAD51 axis similarly protects nonobese diabetic mice from type 1 diabetes through expansion of regulatory B lymphocytes. Journal of Immunology, 198(11), 4255-4267. https://journals.aai.org/jimmunol/article/198/11/4255/106029
Zhu, Q., Wang, J., Zhang, L., Bian, W., Lin, M., Xu, X., & Zhou, X. (2019). LCK rs10914542-G allele associates with type 1 diabetes in children via T cell hyporesponsiveness. Nature Communications, 10(1), 2573. https://doi.org/10.1038/s41390-019-0436-2
Gimenez, C. A., Ielpi, M., Mutto, A., Grosembacher, L., Argibay, P., & Pereyra-Bonnet, F. (2016). CRISPR-on system for the activation of the endogenous human INS gene. Gene Therapy, 23, 543-547. https://doi.org/10.1038/gt.2016.28
Sanchez-Leon, S., Gil-Humanes, J., Ozuna, C. V., Gimenez, M. J., Sousa, C., Voytas, D. F., & Barro, F. (2018). Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnology Journal, 16(4), 902-910. https://doi.org/10.1111/pbi.12837
Çerçi, B., Uzay, I. A., Kara, M. K., & Dinçer, P. (2023). Clinical trials and promising preclinical applications of CRISPR/Cas gene editing. Life Sciences, 312, 121204. https://doi.org/10.1016/j.lfs.2022.121204